Valve-regulated lead-acid batteries (VRLA batteries) avoid the decomposition of water in the electrolyte in the following ways:
- Oxygen cycle principle: At the end of charging, the oxidation of divalent lead on the positive plate and the oxidation of water form a competitive reaction. When the positive plate is charged to about 95%, oxygen begins to precipitate. The precipitated oxygen diffuses to the surface of the negative plate through the small holes of the AGM diaphragm or the holes of the silica gel, and regenerates lead sulfate and water with the lead element and sulfuric acid on the negative plate, avoiding the loss of water. This process also inhibits the generation of hydrogen on the surface of the negative plate.
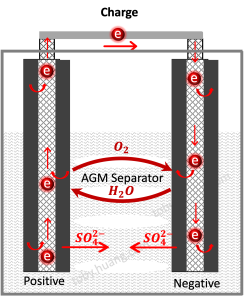
- Sealed design: VRLA batteries use a sealed design to prevent water in the electrolyte from evaporating and outside air from entering the battery, thereby reducing water decomposition and loss.
- Electrolyte adsorption materials: VRLA batteries use highly adsorbable electrolyte adsorption materials, such as glass fiber diaphragms. These materials can adsorb electrolytes, maintain electrolyte stability, and reduce water decomposition.
- Low current charging: VRLA batteries use low current charging during charging to reduce the decomposition of water in the electrolyte. Low current charging can lower the temperature inside the battery and reduce water evaporation and decomposition.
- Additives: Adding some special additives to the electrolyte, such as barium sulfate and strontium sulfate, can improve the stability of the electrolyte and reduce the decomposition of water.
Through the above methods, the valve-regulated lead-acid battery can effectively avoid the decomposition of water in the electrolyte and extend the service life of the battery. If you have more questions or need further explanation, please feel free to let me know!