Electrochemical Impedance Spectroscopy (EIS) is a powerful analytical technique widely used in electrochemical systems. EIS analyzes the kinetic characteristics of electrochemical systems by applying a small sinusoidal AC voltage at different frequencies and measuring the system’s current response. It can provide rich information to help study material and interface processes, such as electrode reaction kinetics, electrode material conductivity, ion migration characteristics in electrolytes, and more.
The core of EIS is to separate different processes in the system through impedance. Each process in an electrochemical system, including charge transfer, diffusion, and double-layer capacitance, has different frequency response characteristics. The low-frequency region is usually associated with diffusion processes, while the high-frequency region reflects charge transfer and double-layer capacitance effects. Thus, by measuring impedance at different frequencies, these processes can be distinguished.
Significance of the High-Frequency Region
In the high-frequency region, fast processes in the electrochemical system are typically reflected, mainly involving the double-layer capacitance (C_dl) and solution resistance (R_s). The solution resistance represents the resistance of the electrolyte solution to the flow of current and is usually a constant value that does not change with frequency. The double-layer capacitance is formed by charge accumulation at the electrode-electrolyte interface and typically exhibits capacitive behavior. High-frequency data can help study the properties of the electrode surface and the electrolyte.
In the frequency response analysis of a battery, particularly during Electrochemical Impedance Spectroscopy (EIS) testing, the high-frequency region typically shows a small semicircular or arc-shaped feature. This high-frequency impedance characteristic provides important information, primarily related to the fast dynamic processes between the electrode materials, electrolyte, and their interfaces within the battery. Below are common curve features in the high-frequency region and their corresponding physical meanings:
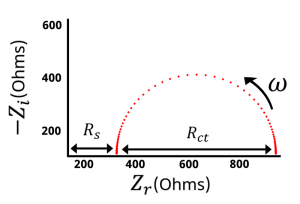
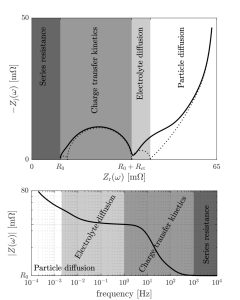
1. Electrolyte resistance (high-frequency intercept): At very high frequencies (usually in the range of several hundred kHz to MHz), the ohmic resistance of the electrolyte dominates. On the impedance graph, a point can be seen intercepting the real axis, corresponding to the electrolyte’s internal resistance (Rs). This point typically reflects the ion conductivity within the electrolyte.
2. Charge transfer resistance and double-layer capacitance (mid-high frequency semicircle): At relatively high frequencies (typically in the kHz to hundreds of kHz range), a semicircle appears in the Nyquist plot. This semicircle reflects the combined effects of the charge transfer resistance at the electrode/electrolyte interface and the double-layer capacitance. The size of the semicircle is related to the charge transfer resistance (Rct), with larger semicircles indicating slower charge transfer processes.
3. Inductive effects (rising trend at very high frequencies): At extremely high frequencies (beyond the MHz range), if inductive components (such as wires or electrodes) are present in the testing system, the impedance plot may exhibit a rising trend, reflecting inductive effects.
- In summary, the high-frequency region of a battery impedance plot typically shows:
- An intercept point (representing the electrolyte resistance),
- A semicircle (indicating charge transfer resistance and double-layer capacitance),
- A possible inductive effect (at very high frequencies).
Significance of the Mid-Frequency Region
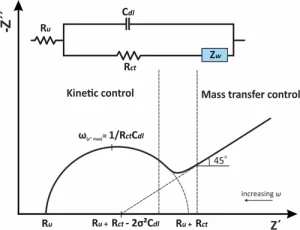
The mid-frequency region typically reflects the charge transfer kinetics of the electrochemical system. This process is associated with the Faradaic reactions on the electrode surface, where electrons are transferred across the electrode-electrolyte interface. In this frequency range, the charge transfer resistance (R_ct) becomes the dominant factor. The smaller the R_ct, the faster the electrochemical reaction rate.
Impedance characteristics in the mid-frequency region help in studying the efficiency of charge transfer in catalysts and electrode materials, as well as the rate of interfacial reactions. In a Nyquist plot, this region often shows a semicircle, with the diameter representing the charge transfer resistance. A smaller semicircle indicates lower charge transfer resistance and faster interfacial reactions.
Significance of the Low-Frequency Region
The low-frequency response typically reflects slower processes such as ion diffusion or mass transport limitations within the electrode material. This phenomenon is often described by Warburg impedance, which appears as an inclined line in the low-frequency region of a Nyquist plot.
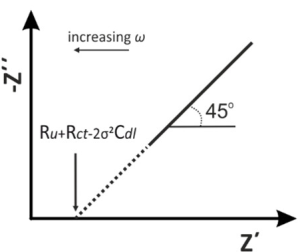
In systems like lithium-ion batteries, the low-frequency response can be used to analyze ion diffusion behavior within the electrode material. Greater diffusion impedance indicates slower ion transport and longer diffusion paths within the electrode. As the frequency decreases, reaction time increases, and diffusion limitations become more apparent. Therefore, the impedance characteristics in the low-frequency region are critical for studying mass transport limitations and diffusion coefficients.
Summary of Frequency Regions
- High-Frequency Region: Reflects solution resistance and double-layer capacitance. Primarily used to study the electrode/electrolyte interface and the conductivity of the electrolyte.
- Mid-Frequency Region: Mainly related to charge transfer processes, reflecting the electrochemical activity of electrode materials and the rate of interfacial reactions.
- Low-Frequency Region: Involves diffusion and mass transport processes, used to analyze ion transport, diffusion impedance, and slow processes within the material.
Applications
EIS is widely used in various fields due to its ability to distinguish between different physical and chemical processes. For example, in the study of lithium-ion batteries, supercapacitors, and fuel cells, EIS is used to evaluate the electrochemical performance of electrode materials, determine internal resistance, polarization impedance, and ion diffusion coefficients. In corrosion studies, EIS helps assess the corrosion resistance of coatings and metals. Additionally, EIS is a crucial tool in the evaluation of biosensors, corrosion-protective coatings, electroplating, and catalysts.
By systematically analyzing the high, mid, and low-frequency regions, researchers can gain a comprehensive understanding of the entire electrochemical process, enabling material optimization, interface design, and performance improvement of systems.